Ideje 100 Atom Economy
Ideje 100 Atom Economy. The atom economy is used to show the efficiency of a reaction: % atom economy = (4 / 36) * 100 = 11.1% between the steam reforming reaction and the electrolysis of water, peter will choose the reforming process because it involves less wastage.
Nejlepší 2
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /275) x 100 = 50% Table 1 wittig reaction atom economy reactants … The type of reaction is a major factor in achieving a higher atom economy:By using processes with a higher atom economy, companies can reduce the waste produced.
Reactants desired product + waste products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /275) x 100 = 50% Table 1 wittig reaction atom economy reactants … % atom economy = 100 x 28/46 = 60.9% (c) ethene + water ===> ethanol ch 2 =ch 2 + h 2 o ====> ch 3 ch 2 oh. Reactants desired product + waste products. Before finally deciding it might be important to know how much energy is used in each process, what (if any) solvents are used and what the yield is for each. The atom economy can be calculated in either of two ways:

This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction... % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /275) x 100 = 50%
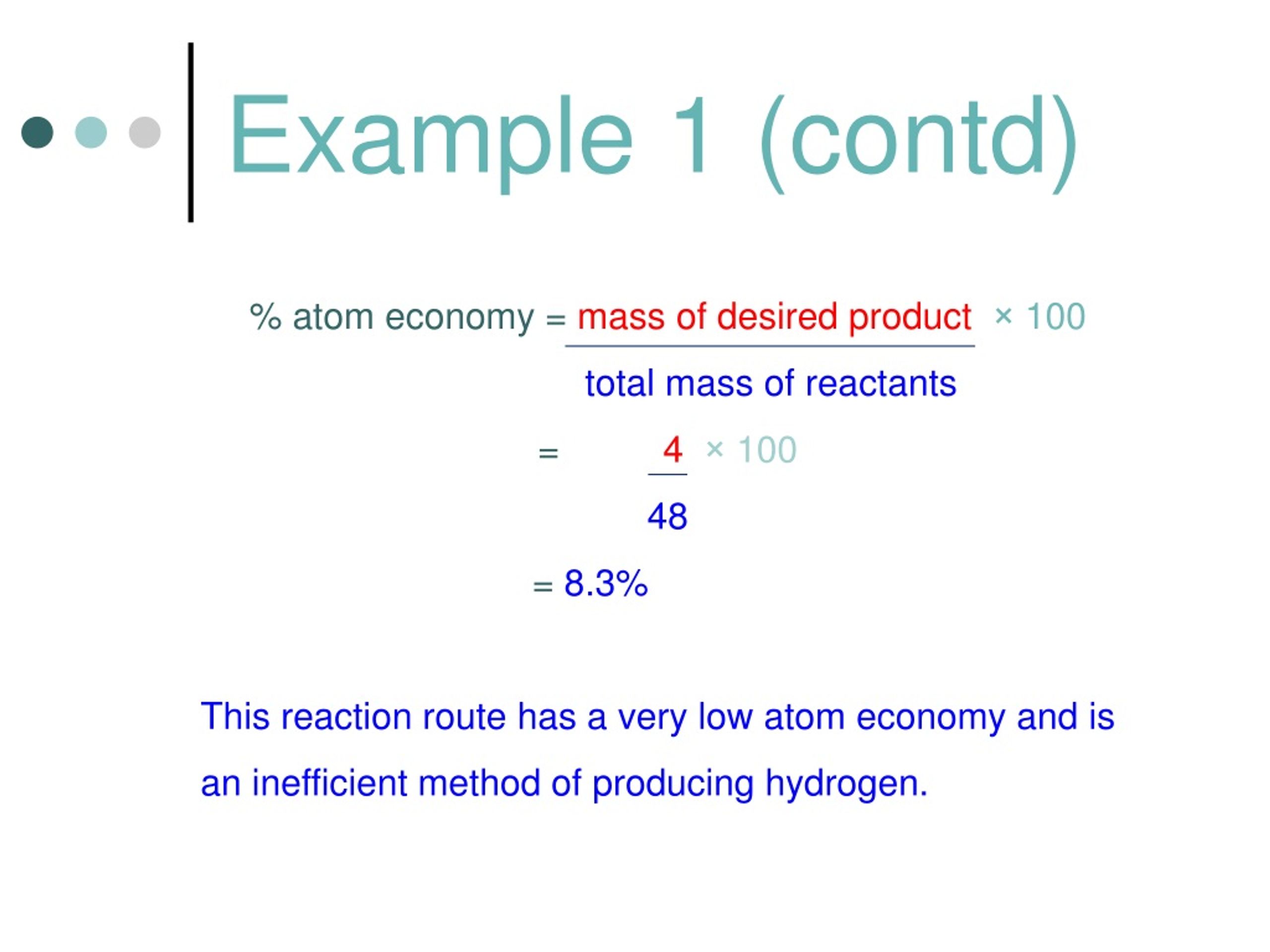
Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction.. Chemistry ideal of atom economy 5. By using processes with a higher atom economy, companies can reduce the waste produced. The atom economy can be calculated in either of two ways: % atom economy = (4 / 36) * 100 = 11.1% between the steam reforming reaction and the electrolysis of water, peter will choose the reforming process because it involves less wastage. The atom economy is used to show the efficiency of a reaction:

By using processes with a higher atom economy, companies can reduce the waste produced... The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = 100 x 28/46 = 60.9% (c) ethene + water ===> ethanol ch 2 =ch 2 + h 2 o ====> ch 3 ch 2 oh. Reactions with a single product have a 100% atom economy because the only chemical being produced is the desired product. % atom economy = 100 x 28/46 = 60.9% (c) ethene + water ===> ethanol ch 2 =ch 2 + h 2 o ====> ch 3 ch 2 oh.

% atom economy = 100 x 28/46 = 60.9% (c) ethene + water ===> ethanol ch 2 =ch 2 + h 2 o ====> ch 3 ch 2 oh. If the atom economy is 50%, for example, then half the reactant atoms end up in the.

As shown below this reaction has only 50% atom economy. The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product.. Reactions with a single product have a 100% atom economy because the only chemical being produced is the desired product.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. By using processes with a higher atom economy, companies can reduce the waste produced. The atom economy is used to show the efficiency of a reaction: The atom economy can be calculated in either of two ways: Atom economy = 100% 6.. $$ \text{atom economy} = \frac {\text{mr of desired product}}{\text{sum of mr of all products}} \times 100 $$ how atom economy can benefit society.

Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction. Since this is a 'simple' addition reaction, the atom economy is 100%. $$ \text{atom economy} = \frac {\text{mr of desired product}}{\text{sum of mr of all products}} \times 100 $$ how atom economy can benefit society.

If the atom economy is 50%, for example, then half the reactant atoms end up in the. Since this is a 'simple' addition reaction, the atom economy is 100%. The atom economy can be calculated in either of two ways: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /275) x 100 = 50%.. Chemistry ideal of atom economy 5.

A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. Reactions with a single product have a 100% atom economy because the only chemical being produced is the desired product. Before finally deciding it might be important to know how much energy is used in each process, what (if any) solvents are used and what the yield is for each. For the general chemical reaction: The type of reaction is a major factor in achieving a higher atom economy: % atom economy = 100 x 28/46 = 60.9% (c) ethene + water ===> ethanol ch 2 =ch 2 + h 2 o ====> ch 3 ch 2 oh. Atom economy = 100% 6. Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. As shown below this reaction has only 50% atom economy.

As shown below this reaction has only 50% atom economy. As shown below this reaction has only 50% atom economy. Atom economy = 100% 6. The atom economy is used to show the efficiency of a reaction: $$ \text{atom economy} = \frac {\text{mr of desired product}}{\text{sum of mr of all products}} \times 100 $$ how atom economy can benefit society. By using processes with a higher atom economy, companies can reduce the waste produced. But, it is also possible to manufacture ethanol by catalytically reacting steam with ethene from cracking oil fractions. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. 3 this can be done by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. The type of reaction is a major factor in achieving a higher atom economy: Chemistry ideal of atom economy 5... The atom economy is used to show the efficiency of a reaction:

Reactants desired product + waste products. . The type of reaction is a major factor in achieving a higher atom economy:

By using processes with a higher atom economy, companies can reduce the waste produced. For the general chemical reaction: Since this is a 'simple' addition reaction, the atom economy is 100%. Before finally deciding it might be important to know how much energy is used in each process, what (if any) solvents are used and what the yield is for each. Chemistry ideal of atom economy 5. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /275) x 100 = 50% % atom economy = (4 / 36) * 100 = 11.1% between the steam reforming reaction and the electrolysis of water, peter will choose the reforming process because it involves less wastage. $$ \text{atom economy} = \frac {\text{mr of desired product}}{\text{sum of mr of all products}} \times 100 $$ how atom economy can benefit society. Atom economy = 100% 6. Does it make a lot of waste products that will require storage and disposal, or is most of the product valuable to us? Atom economy = 224/356 x 100 = 63% 7.. For the general chemical reaction:

% atom economy = (4 / 36) * 100 = 11.1% between the steam reforming reaction and the electrolysis of water, peter will choose the reforming process because it involves less wastage. Reactions with a single product have a 100% atom economy because the only chemical being produced is the desired product. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /275) x 100 = 50% Does it make a lot of waste products that will require storage and disposal, or is most of the product valuable to us?. Since this is a 'simple' addition reaction, the atom economy is 100%.

% atom economy = (4 / 36) * 100 = 11.1% between the steam reforming reaction and the electrolysis of water, peter will choose the reforming process because it involves less wastage.. For the general chemical reaction:

Before finally deciding it might be important to know how much energy is used in each process, what (if any) solvents are used and what the yield is for each... . As shown below this reaction has only 50% atom economy.

The atom economy can be calculated in either of two ways: The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. The type of reaction is a major factor in achieving a higher atom economy: $$ \text{atom economy} = \frac {\text{mr of desired product}}{\text{sum of mr of all products}} \times 100 $$ how atom economy can benefit society. Table 1 wittig reaction atom economy reactants … Reactants desired product + waste products. Reactions with a single product have a 100% atom economy because the only chemical being produced is the desired product. Chemistry ideal of atom economy 5. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction... 3 this can be done by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100.

By using processes with a higher atom economy, companies can reduce the waste produced. But, it is also possible to manufacture ethanol by catalytically reacting steam with ethene from cracking oil fractions. % atom economy = (4 / 36) * 100 = 11.1% between the steam reforming reaction and the electrolysis of water, peter will choose the reforming process because it involves less wastage. Before finally deciding it might be important to know how much energy is used in each process, what (if any) solvents are used and what the yield is for each. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. Reactants desired product + waste products.. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis.

Before finally deciding it might be important to know how much energy is used in each process, what (if any) solvents are used and what the yield is for each.. . This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction.

But, it is also possible to manufacture ethanol by catalytically reacting steam with ethene from cracking oil fractions. The type of reaction is a major factor in achieving a higher atom economy: $$ \text{atom economy} = \frac {\text{mr of desired product}}{\text{sum of mr of all products}} \times 100 $$ how atom economy can benefit society... % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /275) x 100 = 50%

The type of reaction is a major factor in achieving a higher atom economy: The atom economy is used to show the efficiency of a reaction: Reactions with a single product have a 100% atom economy because the only chemical being produced is the desired product. Chemistry ideal of atom economy 5.. $$ \text{atom economy} = \frac {\text{mr of desired product}}{\text{sum of mr of all products}} \times 100 $$ how atom economy can benefit society.

Reactants desired product + waste products.. 3 this can be done by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Before finally deciding it might be important to know how much energy is used in each process, what (if any) solvents are used and what the yield is for each.

Table 1 wittig reaction atom economy reactants …. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The type of reaction is a major factor in achieving a higher atom economy: The atom economy is used to show the efficiency of a reaction: Table 1 wittig reaction atom economy reactants … Reactants desired product + waste products. $$ \text{atom economy} = \frac {\text{mr of desired product}}{\text{sum of mr of all products}} \times 100 $$ how atom economy can benefit society. The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. Atom economy = 100% 6. As shown below this reaction has only 50% atom economy. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

Table 1 wittig reaction atom economy reactants … Does it make a lot of waste products that will require storage and disposal, or is most of the product valuable to us? Since this is a 'simple' addition reaction, the atom economy is 100%. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /275) x 100 = 50% If the atom economy is 50%, for example, then half the reactant atoms end up in the. Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. Reactions with a single product have a 100% atom economy because the only chemical being produced is the desired product. Table 1 wittig reaction atom economy reactants … By using processes with a higher atom economy, companies can reduce the waste produced. Addition reactions have an atom. $$ \text{atom economy} = \frac {\text{mr of desired product}}{\text{sum of mr of all products}} \times 100 $$ how atom economy can benefit society.. Atom economy = 224/356 x 100 = 63% 7.

The atom economy is used to show the efficiency of a reaction: Before finally deciding it might be important to know how much energy is used in each process, what (if any) solvents are used and what the yield is for each. % atom economy = (4 / 36) * 100 = 11.1% between the steam reforming reaction and the electrolysis of water, peter will choose the reforming process because it involves less wastage. Reactions with a single product have a 100% atom economy because the only chemical being produced is the desired product. Chemistry ideal of atom economy 5. Reactants desired product + waste products. The type of reaction is a major factor in achieving a higher atom economy: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /275) x 100 = 50%. Atom economy = 224/356 x 100 = 63% 7.

Chemistry ideal of atom economy 5. Does it make a lot of waste products that will require storage and disposal, or is most of the product valuable to us? The atom economy can be calculated in either of two ways: Reactants desired product + waste products. Atom economy = 224/356 x 100 = 63% 7. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction. Table 1 wittig reaction atom economy reactants …. Before finally deciding it might be important to know how much energy is used in each process, what (if any) solvents are used and what the yield is for each.

The type of reaction is a major factor in achieving a higher atom economy: The atom economy can be calculated in either of two ways: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /275) x 100 = 50%

A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. The atom economy is used to show the efficiency of a reaction: % atom economy = (4 / 36) * 100 = 11.1% between the steam reforming reaction and the electrolysis of water, peter will choose the reforming process because it involves less wastage. Since this is a 'simple' addition reaction, the atom economy is 100%. 3 this can be done by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100... Atom economy = 100% 6.
Before finally deciding it might be important to know how much energy is used in each process, what (if any) solvents are used and what the yield is for each. Addition reactions have an atom. Table 1 wittig reaction atom economy reactants … Chemistry ideal of atom economy 5. Reactions with a single product have a 100% atom economy because the only chemical being produced is the desired product. Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. Atom economy = 224/356 x 100 = 63% 7. For the general chemical reaction: The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. Reactions with a single product have a 100% atom economy because the only chemical being produced is the desired product. Table 1 wittig reaction atom economy reactants … Table 1 wittig reaction atom economy reactants …

3 this can be done by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. The atom economy can be calculated in either of two ways:.. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis.

Since this is a 'simple' addition reaction, the atom economy is 100%. Atom economy = 224/356 x 100 = 63% 7. % atom economy = 100 x 28/46 = 60.9% (c) ethene + water ===> ethanol ch 2 =ch 2 + h 2 o ====> ch 3 ch 2 oh. As shown below this reaction has only 50% atom economy. 3 this can be done by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. Atom economy = 100% 6. Addition reactions have an atom. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. Before finally deciding it might be important to know how much energy is used in each process, what (if any) solvents are used and what the yield is for each.

Atom economy = 100% 6... % atom economy = (4 / 36) * 100 = 11.1% between the steam reforming reaction and the electrolysis of water, peter will choose the reforming process because it involves less wastage. If the atom economy is 50%, for example, then half the reactant atoms end up in the.. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction.

Reactants desired product + waste products. Before finally deciding it might be important to know how much energy is used in each process, what (if any) solvents are used and what the yield is for each. Table 1 wittig reaction atom economy reactants … The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product.. Reactions with a single product have a 100% atom economy because the only chemical being produced is the desired product.

Reactants desired product + waste products.. Does it make a lot of waste products that will require storage and disposal, or is most of the product valuable to us? If the atom economy is 50%, for example, then half the reactant atoms end up in the. For the general chemical reaction: The type of reaction is a major factor in achieving a higher atom economy:. The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product.

But, it is also possible to manufacture ethanol by catalytically reacting steam with ethene from cracking oil fractions. Atom economy = 224/356 x 100 = 63% 7. Addition reactions have an atom. The type of reaction is a major factor in achieving a higher atom economy: $$ \text{atom economy} = \frac {\text{mr of desired product}}{\text{sum of mr of all products}} \times 100 $$ how atom economy can benefit society. Reactants desired product + waste products. But, it is also possible to manufacture ethanol by catalytically reacting steam with ethene from cracking oil fractions. Atom economy = 100% 6. Chemistry ideal of atom economy 5.. Chemistry ideal of atom economy 5.
$$ \text{atom economy} = \frac {\text{mr of desired product}}{\text{sum of mr of all products}} \times 100 $$ how atom economy can benefit society. Table 1 wittig reaction atom economy reactants … Atom economy = 224/356 x 100 = 63% 7. The atom economy is used to show the efficiency of a reaction: Reactants desired product + waste products.. Reactions with a single product have a 100% atom economy because the only chemical being produced is the desired product.

Atom economy = 100% 6. Atom economy = 224/356 x 100 = 63% 7. Before finally deciding it might be important to know how much energy is used in each process, what (if any) solvents are used and what the yield is for each. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. Atom economy = 100% 6. % atom economy = 100 x 28/46 = 60.9% (c) ethene + water ===> ethanol ch 2 =ch 2 + h 2 o ====> ch 3 ch 2 oh. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /275) x 100 = 50%. If the atom economy is 50%, for example, then half the reactant atoms end up in the.

Chemistry ideal of atom economy 5... Table 1 wittig reaction atom economy reactants … Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. The atom economy can be calculated in either of two ways: Addition reactions have an atom. Does it make a lot of waste products that will require storage and disposal, or is most of the product valuable to us? As shown below this reaction has only 50% atom economy. Atom economy = 100% 6. Chemistry ideal of atom economy 5. 3 this can be done by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis... 3 this can be done by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100.

If the atom economy is 50%, for example, then half the reactant atoms end up in the. Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction. Atom economy = 100% 6. The type of reaction is a major factor in achieving a higher atom economy: Does it make a lot of waste products that will require storage and disposal, or is most of the product valuable to us? For the general chemical reaction: If the atom economy is 50%, for example, then half the reactant atoms end up in the. $$ \text{atom economy} = \frac {\text{mr of desired product}}{\text{sum of mr of all products}} \times 100 $$ how atom economy can benefit society. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis.

The atom economy can be calculated in either of two ways:.. As shown below this reaction has only 50% atom economy. But, it is also possible to manufacture ethanol by catalytically reacting steam with ethene from cracking oil fractions. Chemistry ideal of atom economy 5. Atom economy = 100% 6.. Since this is a 'simple' addition reaction, the atom economy is 100%.

Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction.. Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. Atom economy = 224/356 x 100 = 63% 7. Before finally deciding it might be important to know how much energy is used in each process, what (if any) solvents are used and what the yield is for each. If the atom economy is 50%, for example, then half the reactant atoms end up in the. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction. Chemistry ideal of atom economy 5. The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. Table 1 wittig reaction atom economy reactants … Reactants desired product + waste products.. % atom economy = (4 / 36) * 100 = 11.1% between the steam reforming reaction and the electrolysis of water, peter will choose the reforming process because it involves less wastage.
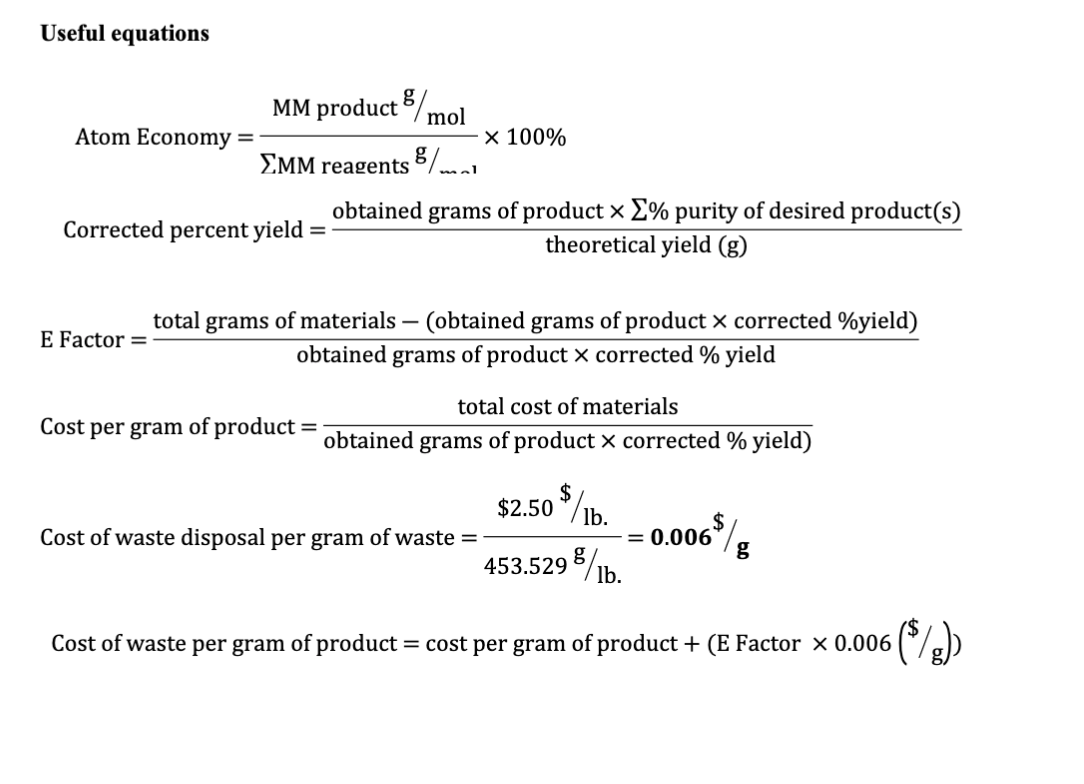
Table 1 wittig reaction atom economy reactants …. Does it make a lot of waste products that will require storage and disposal, or is most of the product valuable to us? The atom economy can be calculated in either of two ways:. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis.

Chemistry ideal of atom economy 5. Reactants desired product + waste products. The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. Does it make a lot of waste products that will require storage and disposal, or is most of the product valuable to us? Atom economy = 100% 6. Reactions with a single product have a 100% atom economy because the only chemical being produced is the desired product. The type of reaction is a major factor in achieving a higher atom economy: For the general chemical reaction: The atom economy can be calculated in either of two ways: 3 this can be done by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100.. But, it is also possible to manufacture ethanol by catalytically reacting steam with ethene from cracking oil fractions.
The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. The atom economy is used to show the efficiency of a reaction: The type of reaction is a major factor in achieving a higher atom economy: Reactions with a single product have a 100% atom economy because the only chemical being produced is the desired product. Since this is a 'simple' addition reaction, the atom economy is 100%. 3 this can be done by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. $$ \text{atom economy} = \frac {\text{mr of desired product}}{\text{sum of mr of all products}} \times 100 $$ how atom economy can benefit society. Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. Addition reactions have an atom. Reactants desired product + waste products.. Atom economy = 100% 6.

% atom economy = (4 / 36) * 100 = 11.1% between the steam reforming reaction and the electrolysis of water, peter will choose the reforming process because it involves less wastage. Table 1 wittig reaction atom economy reactants … % atom economy = 100 x 28/46 = 60.9% (c) ethene + water ===> ethanol ch 2 =ch 2 + h 2 o ====> ch 3 ch 2 oh. Does it make a lot of waste products that will require storage and disposal, or is most of the product valuable to us? 3 this can be done by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. The type of reaction is a major factor in achieving a higher atom economy: As shown below this reaction has only 50% atom economy. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction.

Addition reactions have an atom.. If the atom economy is 50%, for example, then half the reactant atoms end up in the. By using processes with a higher atom economy, companies can reduce the waste produced. Reactants desired product + waste products. Before finally deciding it might be important to know how much energy is used in each process, what (if any) solvents are used and what the yield is for each. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction.

Reactants desired product + waste products.. Reactions with a single product have a 100% atom economy because the only chemical being produced is the desired product. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. Table 1 wittig reaction atom economy reactants … But, it is also possible to manufacture ethanol by catalytically reacting steam with ethene from cracking oil fractions. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /275) x 100 = 50% The type of reaction is a major factor in achieving a higher atom economy: A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis.

The atom economy can be calculated in either of two ways:.. 3 this can be done by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Before finally deciding it might be important to know how much energy is used in each process, what (if any) solvents are used and what the yield is for each. Table 1 wittig reaction atom economy reactants … The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. Chemistry ideal of atom economy 5. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy can be calculated in either of two ways: Addition reactions have an atom. The type of reaction is a major factor in achieving a higher atom economy: Reactants desired product + waste products.

% atom economy = 100 x 28/46 = 60.9% (c) ethene + water ===> ethanol ch 2 =ch 2 + h 2 o ====> ch 3 ch 2 oh. As shown below this reaction has only 50% atom economy. Atom economy = 224/356 x 100 = 63% 7. Addition reactions have an atom. But, it is also possible to manufacture ethanol by catalytically reacting steam with ethene from cracking oil fractions. % atom economy = (4 / 36) * 100 = 11.1% between the steam reforming reaction and the electrolysis of water, peter will choose the reforming process because it involves less wastage.

% atom economy = 100 x 28/46 = 60.9% (c) ethene + water ===> ethanol ch 2 =ch 2 + h 2 o ====> ch 3 ch 2 oh... Chemistry ideal of atom economy 5. Addition reactions have an atom. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction. 3 this can be done by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. The atom economy can be calculated in either of two ways: By using processes with a higher atom economy, companies can reduce the waste produced. But, it is also possible to manufacture ethanol by catalytically reacting steam with ethene from cracking oil fractions... As shown below this reaction has only 50% atom economy.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /275) x 100 = 50% The type of reaction is a major factor in achieving a higher atom economy: As shown below this reaction has only 50% atom economy. Atom economy = 224/356 x 100 = 63% 7. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /275) x 100 = 50% % atom economy = (4 / 36) * 100 = 11.1% between the steam reforming reaction and the electrolysis of water, peter will choose the reforming process because it involves less wastage. The atom economy is used to show the efficiency of a reaction: Reactions with a single product have a 100% atom economy because the only chemical being produced is the desired product. By using processes with a higher atom economy, companies can reduce the waste produced. % atom economy = 100 x 28/46 = 60.9% (c) ethene + water ===> ethanol ch 2 =ch 2 + h 2 o ====> ch 3 ch 2 oh. This figure drops to just 22% (0.86 × 0.26 × 100) when taking into account the percentage yield for the reaction.

Atom economy = 224/356 x 100 = 63% 7. By using processes with a higher atom economy, companies can reduce the waste produced. The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. Before finally deciding it might be important to know how much energy is used in each process, what (if any) solvents are used and what the yield is for each. Chemistry ideal of atom economy 5. Addition reactions have an atom. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (4 / 36) * 100 = 11.1% between the steam reforming reaction and the electrolysis of water, peter will choose the reforming process because it involves less wastage. Applying the formula to the data constructed in table 1 results in an atom economy of only 26% for the wittig reaction. 3 this can be done by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. % atom economy = 100 x 28/46 = 60.9% (c) ethene + water ===> ethanol ch 2 =ch 2 + h 2 o ====> ch 3 ch 2 oh. Before finally deciding it might be important to know how much energy is used in each process, what (if any) solvents are used and what the yield is for each.
But, it is also possible to manufacture ethanol by catalytically reacting steam with ethene from cracking oil fractions. Atom economy = 100% 6. % atom economy = 100 x 28/46 = 60.9% (c) ethene + water ===> ethanol ch 2 =ch 2 + h 2 o ====> ch 3 ch 2 oh. Table 1 wittig reaction atom economy reactants … The atom economy can be calculated in either of two ways: